Summary:
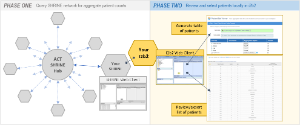
The Accrual to Clinical Trials (ACT) project goal is to create a federated network of National Clinical and Translational Science Award (CTSA) Consortium institutions to significantly increase participant accrual to the nation’s highest priority clinical trials. PHASE ONE consisted of the formation of a SHRINE network of participating institutions in order to query for aggregate patient counts across all sites in real-time. PHASE TWO , as shown in the illustration above, continues the workflow at the local site by enabling the reviewing and selection of patients locally in i2b2.
How-to Guides:
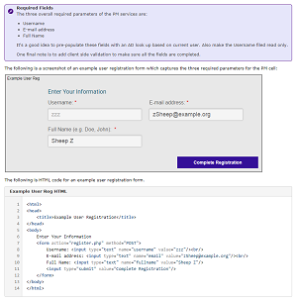
How-to: Create a User Registration AppIn this guide, we demonstrate how you can create a user registration form that will allow users to self register themselves for SHRINE access. The services offered by the PM Cell makes online user registration possible. | |
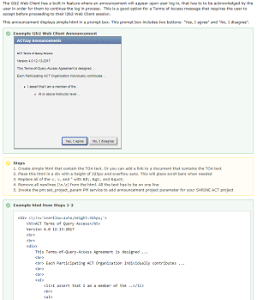
How-to: Automatic Terms of AccessThe SHRINE and i2b2 web clients have a built in feature where an announcement can appear upon user log in. This guide will show how this acknowledgement dialog can be used for the Terms of Access for the ACT project. |
Software:
Phase Two SoftwareIn this guide, we demonstrate how you can create a user registration form that will allow users to self register themselves for SHRINE access. The services offered by the PM Cell makes online user registration possible. |